Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Ingredient Name
- Ingredient Origin
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Ingredients
- Chaga Mushroom Extract
- Technologies
- Product Families
- Chemical Composition
Chaga contains water-soluble pigments that form the chromogenic complex: pterins, as well as humic-like chaga acid (up to 60%), polysaccharides (6 to 8%), lignin, fiber, steroid, pteric compounds (6% to 8%), and 3 yarns acids, free phenols, salts of silicon, iron, aluminum, calcium, magnesium, sodium, zinc, copper, manganese, potassium.
Features & Benefits
- Benefit Claims
- Labeling Claims
- Food Ingredients Features
- Product Background
For a long time, chaga has been used as a remedy against internal tumors in traditional medicine. As the annals indicate, for this purpose it was used as early as the beginning of the twelfth century. Chaga treatment is mentioned in various books of the time. Chaga is widespread throughout the temperate zone of the Northern Hemisphere but does not reach the borders of the birch population range, especially the southern ones.
Outgrowths are found not only on birch trees but also (less often) on alder, mountain ash, ash, elm, in the zone of mixed and deciduous temperate forests, in moderately humid forests and in moderately moist spruce forests with a mixture of birch. The best hosts of the fungus are birches.
- Features and Benefits
- GMO Free
- BSE/TSE free
- Allergen free
Applications & Uses
- Applications
- Food & Nutrition Applications
- Applications
Industry
- Cosmetic
- Pharmaceutical industry
- Food supplements
- Food and drinks
- Feeds
- Healthy nutrition
Properties
- Odor
- Characteristic
- Appearance
- Dark brown fine powder
- Typical Properties
- Physico-Chemical Properties
- Microbiological Values
| Value | Units | Test Method / Conditions | |
| Arsenic Content | max. 2 | ppm | ICP-MS |
| Cadmium Content | max. 1 | ppm | ICP-MS |
| Heavy Metals Content | max. 10 | ppm | ICP-MS |
| Lead Content | max. 3 | ppm | ICP-MS |
| Mercury Content | max. 0.1 | ppm | ICP-MS |
| Polyphenol Content | max. 6 | % | UV Analysis |
| Polysaccharides Content | max. 25 | % | UV Analysis |
| Value | Units | Test Method / Conditions | |
| Ash Content | max. 6 | % | Ph.Eur./USP |
| Bulk Density | 0.3-0.7 | g/ml | Ph.Eur./USP |
| Loss of Drying | max. 5 | % | Ph.Eur./USP |
| Value | Units | Test Method / Conditions | |
| Enterobacteriaceae Content | max. 100 | CFU/g | Ph.Eur./USP |
| Escherichia Coli Content | 1 | gm | Ph.Eur./USP |
| Salmonella | 25 | gm | Ph.Eur./USP |
| Total Plate Count | max. 5000 | CFU/g | Ph.Eur./USP |
| Yeasts and Molds | max. 500 | CFU/g | Ph.Eur./USP |
Regulatory & Compliance
- Statements
- Non Irradiation/Non Ionized Statement
We hereby certify that the product has not been sterilized byionizing radiation at anypoint during the entire manufacturing process and therefore is in full compliance with the relevant legislated regulations (Directive 1999/2/CE). - BSE/TSE Statement
We hereby certify that the product contains no ingredients of ruminant origin and no materials derived from, or exposed to ruminants affected byor under quarantine for Transmitting Transmissible Spongiform Encephalopathy(TSE)/ Bovine Spongiform Encephalopathy (BSĖ) and it is conform to the EU legislation 999/2001. - Packaging Statement
We hereby certify that the packing used is in compliance with: Commission Regulation (EC)No 1935/2004 and Regulation (EU) No 10/2011 regarding packaging materials and articles intended to come into contact with food. - Pesticide Statement
We hereby certify, basis on our actual knowledge of production process, raw materials and equipment used potential pesticide residues in the above-mentioned product comply with the European legislation on pesticide residues, esp. Regulation (EC) No. 396/2005. - Residual Solvent Statement
We hereby certify that the product meets with: Directive E 2010/59/EU of 26 August 2010 amending Directive 2009/32/EC ofthe European Parliamentand of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. - Contaminant Statement
This is to certify that the product meets with:
REGULATION (EC) No. 1881/2006 and subsequent amendments as concerns the maximum level admitted of the following contaminants:- Aflatoxin B1
- Aflatoxins B1 + B2 + G1 + G2
- Ochratoxin
- Melamine
REGULATION (EC) No. 1933/2015 amending regulation (EC) No. 1881/2006 as regards maximum levels for polycyclic arom atic hydrocarbons, in particular:
- Maximum level of 10 ug/kg of benzo(a)pyrene
- 50 ug/kg for the sum of PAH4 (benzo(a)pyrene, chrysene, benz(a)anthracene and benzo(b)fluoranthene) in food supplements.
- Nanomaterial Statement
According to the definition of “nanomaterials" of the EU Regulation (EU) No. 1169/2011 of the European parliament and of the council of 25 October 2011 on the provision of food information to consumers, we hereby attest that no nanomaterials are used in the formulation or in the packaging material of the product. - Anti-Doping Statement
As per the list of 2017 and subsequent amendments of the world anti-doping agency, the ingredient is not a doping substance and not a combination of doping substances. The ingredient does not contain any doping substance. The ingredient does not result from a doping substance. - Expected Usage
The expected usage ofthis product is its incorporation as an ingredientin the food industryor the pharmaceutical industry.
- Non Irradiation/Non Ionized Statement
- Allergen Decleration EU
Allergens Direct Incorporation Cross Contamination Presence on the production line Presence on the production workshop Presence on the production factory Cereals containing gluten (1) and
products thereofNO NO NO NO Crustaceans and products
there ofNO NO NO NO Eggs and products thereof NO NO NO NO Fish and products thereof (2) NO NO NO NO Peanuts and products there of NO NO NO NO Soybeans and products there of
(3)NO NO NO NO Milk and products thereof
(including lactose) (4)NO NO NO NO Nuts (5) or products thereof NO NO NO NO Celery and products there of NO NO NO NO Mustard and products there of NO NO NO NO Sesame seeds and products
there ofNO NO NO NO Sulfur dioxide and sulphites at
concentrations of more than 10
mg/kg or 10 mg/l expressed as
SO2NO NO NO NO Lupines and products thereof NO NO NO NO Mollusk and product thereof NO NO NO NO (1) Cereals which contain gluten (i.e.w heat, rye, barley, oats, spelt, kamut or their hybridized strains) except: w heat-based glucose syrups including dextrose, wheat-based maltodextrins, glucose syrups based on barley, cereals used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beverages.
(2) Except: fish gelatine used as carrier for vitamin or carotenoid preparations, fish gelatine or singlass used as fining ag ent in beer and wine.
(3) Except fully refined soybean oil and fat, natural mixed tocopheols (E306), natural D-alpha tocopherol, natural D-alpha tocopherol acetate, natural D-alpha tocopherol succinate from soybean sources;vegetable oils derived phytosterols and phytosterolesters from soybean sources; plant stanol es ter producted from vegetable oil sterols from soybean sources.
(4) Except when used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beverages, lac tito.
(5) Almond (Amydalus communis L.) hazelnuts (Corylus avellana), walnut (Juglans regia), cashew (Anacardium occidentale), pecan nuts (Carya illinaiesis), brazil nut (Bertholletia excelsa), pistachio nut (Pistacia vera), macadamia nut and queensland nut
(Macadamia terniflora) and products thereof, except nuts used for making distillates or ethyl alcohol of agricultural origin for spirit drinks and other alcoholic beveragesIn accordance with the directive 1169/2011 EC.
- GMO Declaration
Present GM-Origin Cotton No Not-Applicable Maize No Not-Applicable Potato No Not-Applicable Rape No Not-Applicable Soya No Not-Applicable Sugarbeet No Not-Applicable Tomato No Not-Applicable Wheat No Not-Applicable The following ingredients produced from genetically modified organisms are present in the product: None
In accordance with the regulation (EC) No 1829/2003 and (EC) No 1830/2003 of the European Parliament
Technical Details & Test Data
- Plant Information
- Scientific name : Inonotus obliquus (Ach. ex Pers.) Pilat
- Synonyms : Polyporus obliquus (Ach. ex Pers.) Fr. Fomes obliquus (Ach. ex Pers.) Cooke
- Botanical family : Hymenochaetaceae
- Common names : Chaga, birch conk
- Geographical origin : Russian Federation, Siberia
Harvest
Period (month/s or season) Spring / autumn Method (manual/mechanical) Manual Used part Sclerotium Type of culture (wild/cultivated) Wild Plant growth stage Sclerotium diameter at least 20 cm, the age is at least 7-10 years After Harvest
Crushed Yes Cleaned Yes Dried Electric oven Additional Information
Treatments before/after harvest No / No Identification Botanical and chemical (Pharm. Russian Federation "Chaga") Storage In dry and cool well-ventilated place away from light - Extraction Details
Extraction method Solvent extraction Solvent(s) Water Drying method Spray Carrier/ excipient No Ratio 8:1 - Product Flow Chart
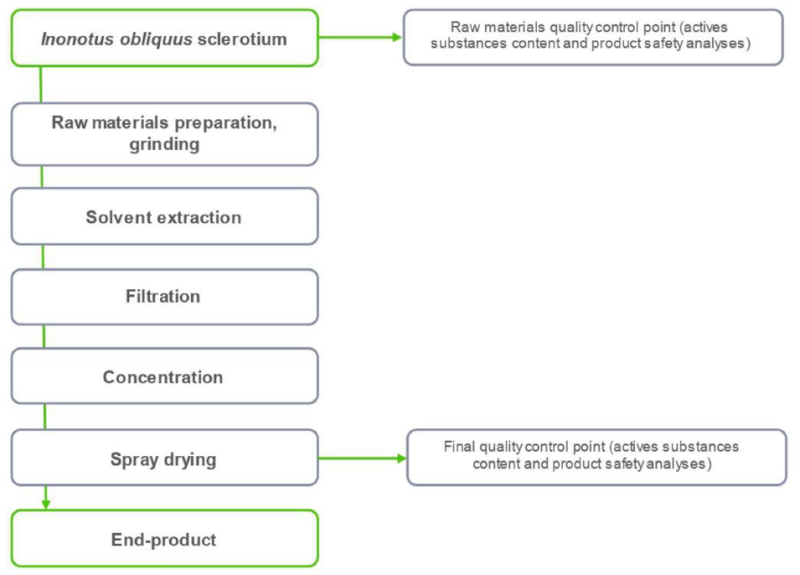
- Manufacturing Process
We have built a full production cycle - from the search and collection of raw materials, the manufacture of the product to the delivery of the order to the client's territory. This guarantees you stable prices, stable quality and continuous deliveries. We focus on the needs of our customers and, upon request, carry out additional analyzes, develop the necessary documentation. and we produce extracts and powders with the characteristics you need.
Verification : We check raw materials and final products with the help of laboratory tests at every stage from raw material preparation to finished extracts and powders. The supplied products comply with the EU safety and quality standards.
Safety & Health
- Influence
- General Effects on Body
- It is effective in oncological diseases, especially affecting organs with abundant blood supply - lungs, stomach, as well as in cases excluding radiation therapy and surgical methods.
- It has an antispasmodic effect (relieves spasms of smooth muscles of internal organs)
- It protects liver cells from toxic damage
- It has an immunomodulatory effect (increases immunity), chaga increases the body's resistance to infectious diseases
- It has a diuretic effect (diuretic)
- It has anti-inflammatory properties
For Female Health- It is used to treat tumors of the uterus and ovaries improves insomnia performance
- It is used for infertility treatment and treatment of mastopathy
For Male Health- It is used to improve potency
Impact on the Circulatory System- It lowers blood sugar
- It lowers arterial and venous pressure, reduces the pulse rate, regulates the activity of the cardiovascular system
- It stimulates blood formation (increases the level of leukocytes - cells responsible for immunity)
Gastrointestinal Tract- It normalizes the activity of the gastrointestinal tract and intestinal microflora
- It contributes to the scarring of gastric and duodenal ulcers
Effect on the Skin- It is used to treat skin diseases (such as psoriasis)
- When it is used externally, chaga exhibits an anti-inflammatory, healing and analgesic effect, protects the skin from the harmful effects of the external environment, including fungal and viral infections, relieves swelling and contributes to the restoration of healthy skin.
- Indications & Contrindications
Indications
- Oncological diseases
- Reduced immunity
- Mastopathy
- Gastrointestinal diseases
- Microflora disorders
- Psoriasis and other skin diseases
Contrindications
- Colitis
- Desentery
- Pregnancy
- Penicillin antibiotic treatment
Packaging & Availability
- Packaging Information
- Ingredients are packed into double bags to avoid moisture condensation. During shipment bags are stored into double-double carton boxes.
- 10 kg/carton box
Storage & Handling
- Shelf Life
- 3 years
- Storage and Shelf Life
Stored in a well-closed container in cool and dry place, keep away from direct strong light and heat. Not less than 2 years when properly stored (see COA).