Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
Features & Benefits
- Benefit Claims (Health)
- Features
Supplementing your diet with the ADM Probiotic AD Blend may help support:
- Overall skin health
- Gut microbiome diversity
- Clear & healthy skin
ADM Probiotic AD Blend is the choice ingredient to integrate into dietary supplements or nutritional products targeting skin health. It comes in a bulk powder format and is designed for dietary supplements, specialized in clinical nutrition, with excellent results in dairy and alternative dairy. It has a shelf life of 18 months.
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Properties
- Physical Form
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Test Data
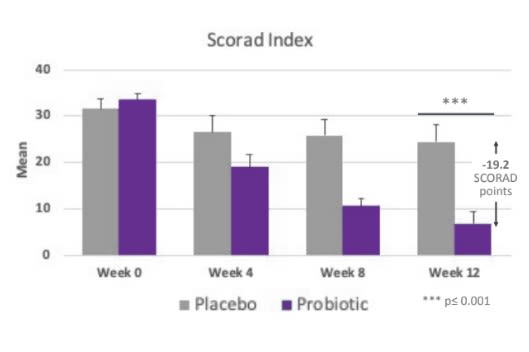
Improvements In Scorad Index
- After 12 weeks, 96% of participants in the ADM Probiotic AD Blend group showed an improvement in the SCORAD index (measured by the SCORAD* index), compared to 46% in the placebo group.
- After 12 weeks of follow-up, the mean reduction in the SCORAD index in the probiotic group was 19.2 points greater than in the control group.
- In relative terms, we observed a reduction in SCORAD of 83% in the probiotic group compared to a reduction of 24% in the placebo group (P < .001).
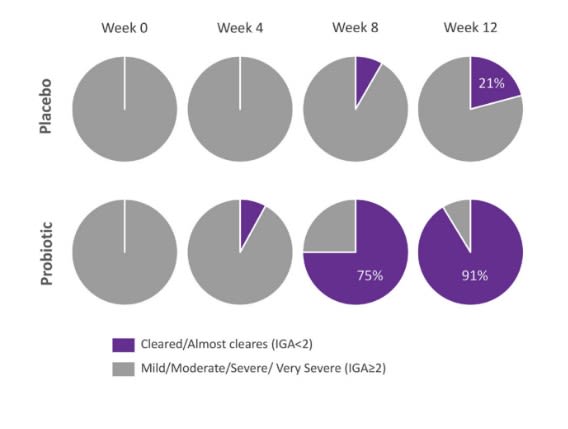
Investigator Global Assessment
Over the 12 week study period, 91% of individuals in the probiotic arm showed improvement to IGA score 0 or 1, compared to 21% in the placebo group.
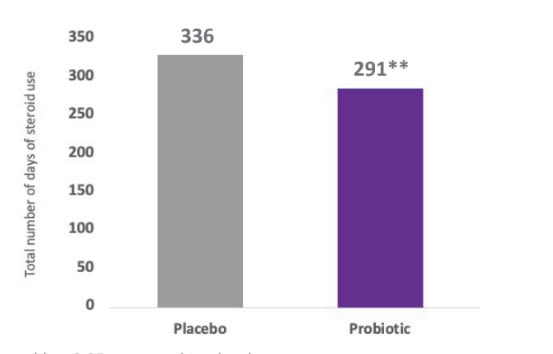
- In this trial, there was a significant reduction in the use of topical steroid in the probiotic group compared to the control group with an odds ratio (OR) of 0.63, indicating a relative risk reduction (RRR) of 37%.
- Reduction in topical steroids use in this trial reached 45 days in the probiotic group compared to placebo.
Packaging & Availability
- Packaging Information
Bulk Powder
Storage & Handling
- Shelf Life
- 18 Months

