Knowde Enhanced TDS
Identification & Functionality
- Ingredient Name
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
Features & Benefits
- Benefit Claims (Health)
- Product Benefits
May help support digestive health
- The strain has shown the properties of increasing the width of the villi of the intestine and the height of the enterocytes in an enteropathy animal model.
- In a pre-clinical setting, it has been shown to decrease the expression of TNF-α in intestinal epithelial cells exposed to gliadins digested by B. longum ES1.
- Has been shown to maintain stability under acidic and bile salt conditions to ensure viability during GI transit.More than 50% survival at pH 2; More than 60% survival at 3% bile salts
May support overall gut health
- In a pre-clinical model, Bifidobacterium longum ES1 significantly reduced levels of the pro-inflammatory agents IFN-γ and TNF-α, associated with tissue damage and inflammation.
- In an in vitro model, Bifidobacterium longum ES1 reduced pro-inflammatory markers and increased the level of the anti-inflammatory cytokine IL-10.
- Bifidobacterium longum ES1 decreased oxidative stress in C. elegans model, increasing its survival under this stress.
In-vitro data suggests gut microbiome modulation
- In vitro, the strain has been shown to inhibit the growth of pathogenic species of the digestive tract, including Clostridium difficile isolates.
- In a clinical trial with children, administration of Bifidobacterium longum ES1 was associated with a significant decrease in the opportunistic pathogen Bacteroides fragilis.
Inhibits growth of C. difficile6 and decreases B. fragilis.
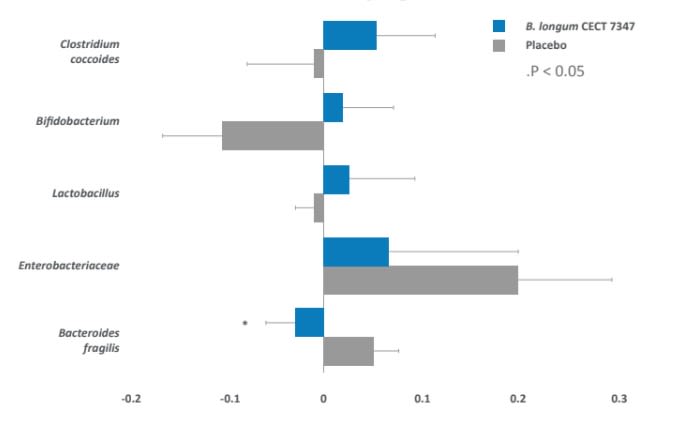
Post-intervention-baseline/baseline numbers of specific bacterial groups (log gene copy number/g stools)
Applications & Uses
- Markets
- Applications
- Food & Nutrition Applications
Properties
- Physical Form
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- Human Clinical Trials
A double-blind, randomized, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum ES1 co-admistered with a gluten-free diet in children with newly diagnosed coeliac disease
Design:
Double-blind, placebo-controlled design. 36 children newly- diagnosed with celiac disease, a disease characterized by intestinal inflammation, intestinal dysbiosis and an altered intestinal structure.
Intervention:
- Bifidobacterium longum ES1
- 1B CFU/day for 12 weeks.
Results:
- Greater height percentile increases in the Bifidobacterium longum ES1 group compared to placebo group (P=0.048).
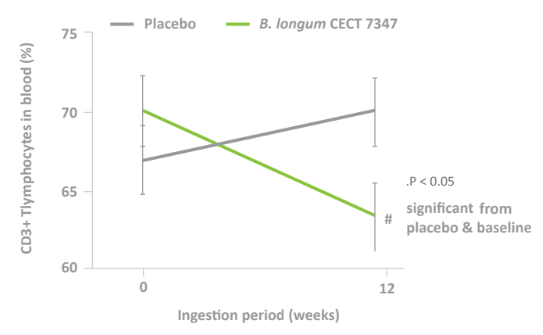
- Decreased peripheral CD3+ T lymphocytes in the Bifidobacterium longum ES1 group compared to placebo (P=0.004).
- Comparison between the groups showed that the administration of Bifidobacterium longum ES1 reduced the numbers of the unwanted bacteria Bacteroides fragilis (P=0.020) and the concentration of secretory IgA in stools (P=0.011) compared to the placebo group.
- A Pilot Study on Non-celiac Gluten Sensitivity
Effects of Bifidobacterium longum ES1 co-administered with a gluten-free diet:
Design:
- Non-randomized, open label study. 30 participants with symptoms attributable to non-celiac gluten sensitivity (NCGS).
Intervention:
- Bifidobacterium longum ES1
- 1B CFU/day for 12 weeks
Results:
A combination of gluten-free diet plus Bifidobacterium longum ES1 resulted in significant improvements in the frequency and intensity of digestive and extra- intestinal episodes:
- Improvements in multiple outcome measures including occasional abdominal pain and swelling, dharrhea and constipation compared to baseline. Furthermore, better stool consistency.

