Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- INCI Name
- Agrochemical Functions
- Cleaning Ingredients Functions
- Cosmetic Ingredients Functions
Features & Benefits
- Benefit Claims
- Labeling Claims
- Agrochemicals Features
Applications & Uses
- Applications
- Application Technique
- Hair Care Applications
- Personal Hygiene Applications
- Use Level
- 0.1 - 0.5%
- Cosmetic Applications
Used for improving the efficiency of formulations and for storage-life stabilization, H-Quest® products are valuable additives in the cosmetic industry. Many ingredients used in cosmetic products are liable to undergo changes during storage due to the catalytic action of trace metal impurities. H-QuestTM products are used to protect against a variety of issues such as, discoloration, change in odor, and various other chemical or physical changes.
- Depilatories - H-Quest® will complex trace metal ions in cosmetic formulations, reducing the oxidation tendency of thiol. The addition of H-Quest® products and proper maintenance of alkalinity provide reproducible and dependable speed of dilapidation. Additionally, it suppresses odor and prevents undesirable coloration of the formula caused by trace metal ions.
- Cold Perm Lotions - Thioglycolic acid and its salts are subject to air oxidation, especially in the presence of various heavy metal salts. H-Quest® products are capable of sequestering many metal ions at pH 9, the pH at which such lotions are employed. The inclusion of H-Quest® products into cold-waving lotions during manufacturing minimizes decomposition of thioglycolate.
- Shampoos - Most shampoos desire formulations to be clear and free of turbidity. Combinations of various sequestering and coupling agents are used to maintain shampoos’ clarity over a wide range of temperatures. Typically, EDTA is used to prevent the formation of calcium and magnesium soaps which can cause turbidity. However, EDTA does not perform well when sequestering ferric and ferrous ions at the low pH of most shampoos. For such situations, H-Quest® products can be implemented.
- Corrosion Inhibition - The beta-isomer of Sodium Glucopheptonate exhibits excellent corrosion-inhibiting properties, particularly for ferrous metals. Low additions of Sodium Glucoheptonate to aqueous solutions offer maximum protection of metal.
- Application Use Levels
Application of Sodium Glucoheptonate in the cosmetic industry is based on the sequestration of trace metal impurities. Use levels are dependent on various factors including the amount of metal ions present and the pH. Typically, a concentration level of 0.1% to 0.5% Sodium Glucoheptonate is effective in odor control in depilatory formulations. However, these figures are only suggested concentrations.
- Metal Cleaning and Etching Applications
Sodium glucoheptonate has many industrial uses as a high performance chelating agent. Its superior performance in the pH range between 9 and 14 is due to its efficiency in forming stable chelates with di- and tri-valent metal ions such as Ca+2, Mg+2, Fe+2, Fe+3, Zn+2, Al+3, etc.
Alkali Cleaning, De-rusting and Paint Stripping of Metals
Sodium glucoheptonate is an effective sequestrant for alkaline earth metals such as calcium and magnesium. It is also an excellent sequestrant for iron under alkaline conditions. Due to its superior sequestering abilities in alkaline solutions, sodium glucoheptonate inhibits the formation of insoluble calcium and magnesium salts. Additionally, it dissolves ferric oxide (rust) films. Laboratory data indicate that sodium glucoheptonate/caustic soda solutions form a protective film on the metal, limiting degradation of the base metal. “After-rusting” tendencies are of lesser severity than those resulting from mineral acid cleaning or standard de-rusting procedures. Solutions of sodium glucoheptonate and caustic soda remove oil, light soil, and phosphate deposits. A sodium glucoheptonate to caustic soda ratio of 1:5 - 1:10 is suggested, with a caustic concentration of 50 - 400 grams per liter of soak tank solution. A temperature range from 70-95°C is recommended. Such solutions are compatible with alkali detergent builders and wetting agents.
Aluminum Etching and Cleaning
Preparation of aluminum for anodizing, electroplating or coating requires thorough cleaning of the surface. Previously, mineral acids were used for surface cleaning, but they vigorously attacked the aluminum surface, causing pitting. Caustic soda solutions served as improved substitutes. Alkali treatment alone, however, does not sufficiently remove the inert protective film of aluminum oxide. Thus, the full brightness potential of the aluminum surface is limited. Futhermore, using caustic-only cleaners for aluminum etching results in the formation of scale on heating coils and tank surfaces. Adding sodium glucoheptonate to caustic soda cleaning solutions greatly aids the process of aluminum etching and cleaning. Sodium glucoheptonate dissolves the aluminum oxide film from the surface and subsequently prevents its redeposition as an oxide scale on heating coils. Optimal caustic concentations vary from 1 -10 oz of alkali per gallon of bath solution, with a 1 - 5% addition of sodium glucoheptonate relative to the dry weight of the alkali.
- Oil Field and Construction Applications
- Sodium glucoheptonate is a sodium salt of polyhydroxymonocarboxylic acid (2,3,4,5,6,7-hexahydroxy-s- heptonic acid). Testing confirms that sodium glucoheptonate is readily biodegradable.
- Sodium glucoheptonate has many industrial uses as a high performance chelating agent. Its superior performance in the pH range between 9 and 14 is due to its efficiency in forming stable chelates with di- and tri-valent metal ions such as Ca+2, Mg+2, Fe+2, Fe+3, Zn+2, Al+3, etc.
- Sodium glucoheptonates have superior hydrolytic stability and sequestration capibility when compared to sodium gluconates in highly alkaline, high temperature baths. Sodium glucoheptonates are also superior to polyphosphates, which hydrolyze and become ineffective under the conditions cited above. Moreover, sodium glucoheptonates are non-corrosive and non-toxic materials.
- Using high purity raw materials in our manufacturing processes, Harcros simultaneously produces both alpha- isomer crystalline product (H-Quest® C-100) and standard liquid sodium glucoheptonate (H-Quest® B105). Harcros also manufactures a 38% calcium glucoheptonate liquid.
Concrete Additives for Oil Field and the Construction Industry
- Additions of small amounts of sodium glucoheptonates to concrete admixtures significantly affects the setting time, resulting in improved plasticity and cohesion of the wet mix. In addition, the presence of sodium glucoheptonate affects the hardening rate of the cement, resulting in increased strength development. Sodium glucoheptonate produces a permanent increase in the cement volume, and inhibits corrosion of the metal used in reinforced concrete. The above features – excellent plasticity, increased compressive strength, and a permanent increase in volume – are achieved with a lower than normal water-to-cement ratio.
- H-Quest® C-100, H-Quest® B105, and 38% calcium glucoheptonate are used in formulating cement retarders. The higher thermal stability of H-Quest® C-100 allows formulated cement retarders to be stable above 400°F. Additionally, calcium glucoheptonate acts as a dispersant for concrete particulates.
Properties
- Appearance
- Light tan crystals
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Solids Content | 99.0 | % | — |
| pH (at 1% DI water) | 8.00 - 10.00 | — | — |
| Specific Gravity | Solid | — | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Eco-Toxicity
- H-Quest® C-100 has recieved DfE approval and been granted a ‘ Full Green Circle'.
- H-Quest® C-100 is readily biodegradable.
Technical Details & Test Data
- Test Data on Agricultural Applications
- Plant vitality relies on sunlight, moisture, and macronutrients in the soil (Nitrogen, Phosphorus & Potassium.).
- Micronutrients like iron, manganese, copper, molybdenum, boron, chromium, zinc, cobalt and magnesium are nature’s catalyst for converting sunlight, nitrogen, phosphorous, and potassium into biomass. High yeild crop production, lush lawns, and healthy ornamentals depend on the plant’s ability to utilize these micronutrients.
- H-Quest Glucoheptonates are eco-friendly products for addressing micronutrient needs. These products are based on the naturally derived sugar, glucose. H-Quest Glucoheptonates bind a wide variety of metals that are critical to plant growth.
- Iron is the workhorse of the micronutrient family - the entire food chain depends on iron for its existence- in plants, iron is essential for chlorophyll production and photosynthesis. Iron is essential to plant vitality. The superior complexing performance of Glucoheptonate over the pH range important to agriculture is apparent. (Fig. 1) The graph below (Fig. 2) compares the chelation of iron with Glucoheptonate and common amino-carboxylates.
- The benefits of Fe-Glucoheptonate are many. Fe-Glucoheptonate has greater stability in the presence of calcium than chelates like EDTA and EDDHA. The extraordinary affinity of Glucoheptonate for iron creates the opportunity to make strongly bound micronutrient complexes. Fe-Glucoheptonate also excels in foliar applications and is used to improve fruit yields and fruit quality.
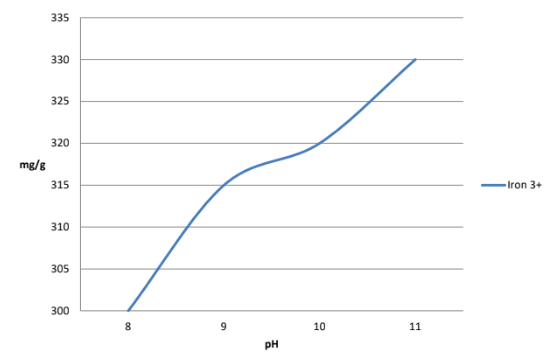
Fig.1 Iron3+ Binding Capacity pH 8-11 (mg/g)
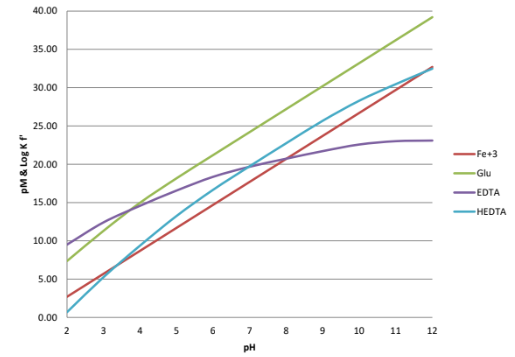
Fig. 2 Glucoheptonate vs EDTA vs HEDTA Fe3+ System 1% Excess Chelant
Storage & Handling
- Storage & Handling Information
This product has excellent storage stability at a variety of temperatures. Storage at room temperature is suggested.

