Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- CAS No.
- 87-99-0
- EC No.
- 201-788-0
- Technologies
- Product Families
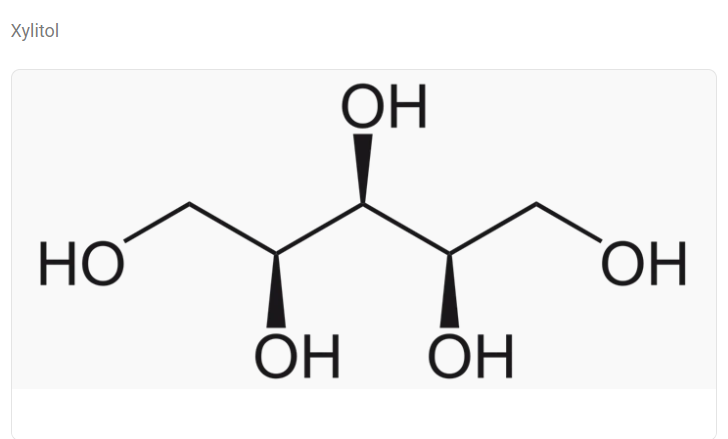
- Chemical Structure
Features & Benefits
- Benefit Claims
Applications & Uses
- Applications
- Dosage Form
- Manufacturing Technology
- Application
- XYLISORB® XTAB 400 / Xylitol granulated with maize dextrin is a direct compression excipient and bulk sweetener. It is used in pharmaceutical and nutraceutical applications including swallowable tablets, effervescent tablets and medicated confectionary.
Properties
- Taste
- Sweet
- Appearance
- Off - white and odourless powder Sweet and cool
- Typical Properties
- Physico-Chemical Properties
- Microbiological Values
- Note
- (*)-Compliance data - Tests not performed
- (**)-Monitoring plan
- Quantitative Composition
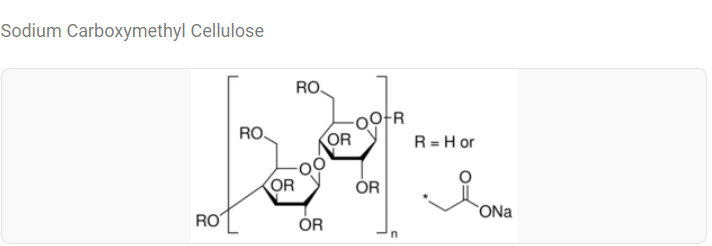
- Approximately 98% of Xylitol / 2% of Sodium Carboxymethyl Cellulose
- Solubility
- Very soluble in water (solubility of xylitol in water is 1 part of xylitol for 1.6 part of water at 20°C)
| Value | Units | Test Method / Conditions | |
| Angle of Repose | 40 | Degree | — |
| Average Mean Particle Diameter | 400 | µm | — |
| Bulk Density | 0.5 - 0.7 | kg/l | — |
| Lead Content (**) | max. 0.5 | mg/kg | — |
| pH Value (50% W/W) | 5.0 - 7.0 | — | — |
| Mean Diameter | approx. 400 | microns | — |
| Melting Temperature (for xylitol) | 92 - 94 | °C | — |
| Na Carboxymethyl Cellulose (on DS) | min. 1 | % | — |
| Particle Size Distribution by Laser Diffraction (dv10 %vol) | 158 | µm | — |
| Particle Size Distribution by Laser Diffraction (dv50 %vol) | 314 | µm | — |
| Particle Size Distribution by Laser Diffraction (dv90 %vol) | 567 | µm | — |
| Powder Flowability | 10.8 | sec | according to Ph.Eur. 2.9.16, 10mm outlow opening |
| Reducing Sugars | max. 0.2 | % | — |
| Residue on 100 microns | min. 90 | % | — |
| Residue on 800 microns | max. 5 | % | — |
| Residue on Ignition (*) | max. 1 | % | — |
| Specific Surface Area | max. 0.2 | m2/g | — |
| Tapped Density | 0.68 | g/cm3 | — |
| True Density | 1.52 | g/cm3 | — |
| Water Content (LOD) | max. 0.5 | % | — |
| Xylitol on DS | min. 98 | % | — |
| Value | Units | Test Method / Conditions | |
| Nickel Content (*) | max. 1 | mg/kg | — |
| Value | Units | Test Method / Conditions | |
| Escherichia coli | Not detected | per gram | — |
| Salmonella (**) | Not detected | per 10g | — |
| Total Aerobic Microbial Count | max. 1000 | CFU/g | Plate count |
| Total Yeasts and Moulds Count | max. 100 | CFU/g | — |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Conformity
- Raw materials
- XYLITOL European Pharmacopeia (1381) and National Formulary from USP-NF
- SODIUM CARBOXYMETHYL CELLULOSE European Pharmacopeia (0472) and National Formulary from USP-NF
Technical Details & Test Data
- Morphology

- Note
- Not intended for use in manufacture of parenteral dosage forms.
- Methods used by Roquette may be the Pharmacopeia methods or alternative validated methods which have been compared to the Pharmacopeia methods.
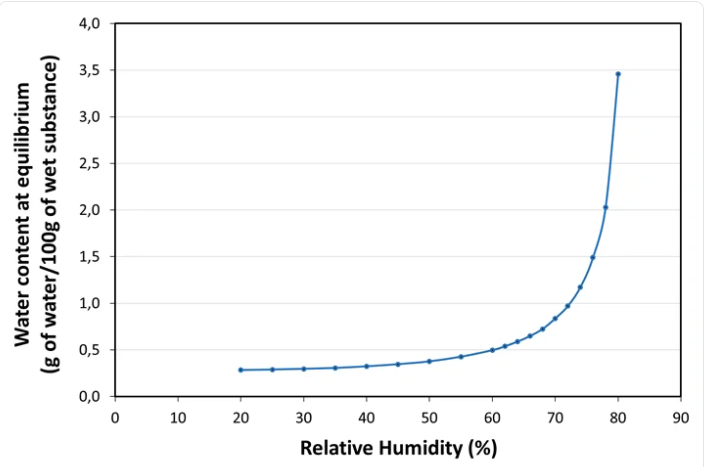
- Water Sorption Isotherm at 20°C
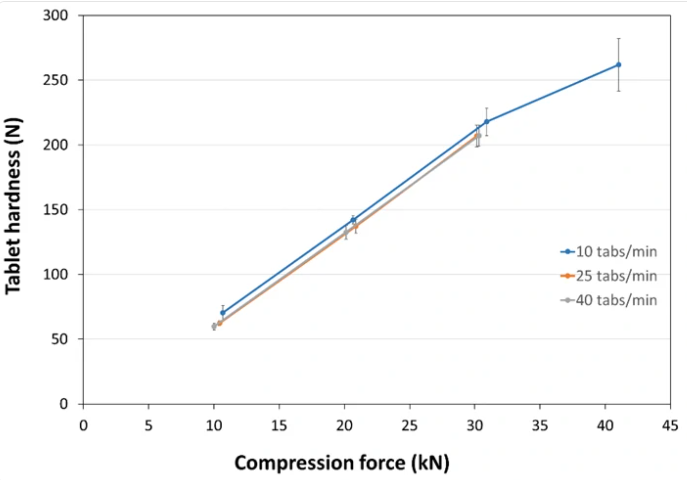
- Compression Behavior
- Innovation Hub
- Innovation Hub by RoquetteLooking for technical support or formulation inspiration? Visit Roquette’s Innovation Hub.
Packaging & Availability
- Packaging Information
- ROQ Product Code: 493404
- Article (SKU) Code: 493404107E
- Package Size & Type: 15 kg PE lined cardboard box
Storage & Handling
- Storage & Shelf Life
- Expiry date Manufacturing date + 5 years, in its unopened packaging.
- The product durability may vary according to packaging type and manufacturing plant. Proper information is shown on labeling and CoA.
- We recommend to preserve the product in its unopened original packaging, preferably protected from wide variations of temperature and humidity.
- Upon opening, use the product as quickly as possible to prevent moisture regain.
- Due to its fine particle size, this product is liable to become compacted.

