Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Technologies
- Product Families
- Chemical Structure
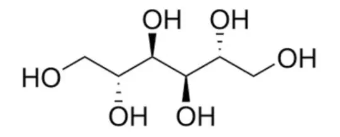
- Morphology

Features & Benefits
- Labeling Claims
Applications & Uses
- Applications
Properties
- Physical Form
- Solubility
- Odor
- Slightly sweet and cooling effect
- Insoluble in
- Ether, Ethanol (96%)
- Physico-Chemical Properties
- Microbiological Values
- Powder Characteristics
| Value | Units | Test Method / Conditions | |
| Loss on Drying | max. 0.5 | %(w/w) | EP, USP-NF, JP |
| Reducing Sugars (as is) | max. 0.1 | %(w/w) | EP, USP-NF, JP |
| Mannitol (on D.S.) | 97.0 - 102.0 | %(w/w) | EP, USP-NF, JP |
| Impurity A : D-sorbitol | max. 2.0 | %(w/w) | EP, USP-NF, JP |
| Sum of Impurities B+C : Maltitol + Isomalt | max. 2.0 | %(w/w) | EP, USP-NF, JP |
| Unspecified Impurities | max. 0.10 | %(w/w) | EP, USP-NF, JP |
| Total Impurities | max. 2.0 | %(w/w) | EP, USP-NF, JP |
| Conductivity | max. 20 | μS/cm | EP, USP-NF, JP |
| Nickel | max. 1 | mg/kg | EP, USP-NF, JP |
| Heavy Metals | max. 5 | mg/kg | JP |
| Melting Point | 165 - 170 | °C | EP, USP-NF, JP |
| Average Mean Particle Diameter | 160.0 | µm | — |
| Particle Size Distribution by Laser Diffraction (dv10) | 90.0 | µm | — |
| Particle Size Distribution by Laser Diffraction (dv50) | 150.0 | µm | — |
| Particle Size Distribution by Laser Diffraction (dv90) | 260.0 | µm | — |
| Laser Particle Size (Part. > 300mm) | max. 25 | % | — |
| Laser Particle Size (Part. > 140mm) | max. 40 | % | — |
| Laser Particle Size (Part. > 30mm) | max. 90 | % | — |
| Average Molecular Weight | 182.2 | g/mol | — |
| Value | Units | Test Method / Conditions | |
| Total Aerobic Microbial Count | max. 1000 | per g | EP, USP-NF |
| Total Yeasts and Molds Count | max. 100 | per g | EP, USP-NF |
| Escherichia Coli | Not detected in 1g | — | EP, USP-NF |
| Salmonella | Not detected in 10g | — | EP |
| Value | Units | Test Method / Conditions | |
| Powder Flowability (10mm outflow opening) | 4.0 | seconds | according to Ph.Eur. 2.9.16 |
| Bulk Density | 0.63 | g/cm³ | — |
| Tapped Density | 0.75 | g/cm³ | — |
| True Density | 1.514 | g/cm³ | — |
| Specific Surface Area | 1.3 | m²/g | — |
| Angle of Repose | 27.0 | ° | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
Technical Details & Test Data
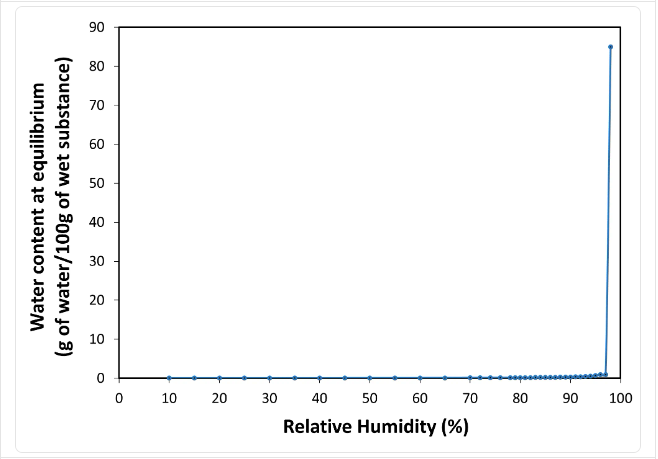
- Water Sorption Isotherm at 20°C
- Tablet Hardness
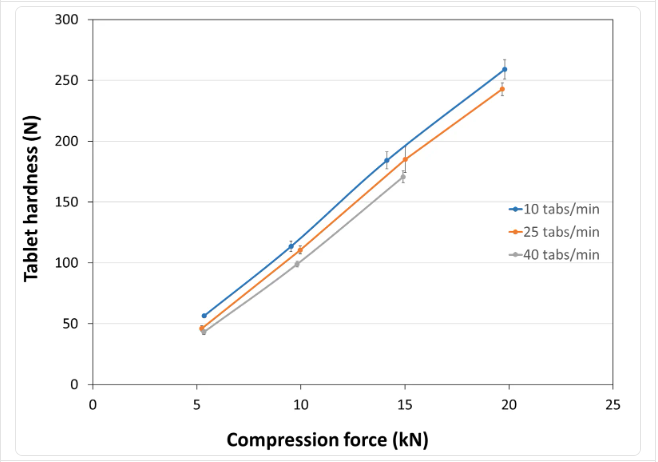
- Experimental Conditions for Compression Behavior
- Tablet Press: STYLONE EVO
- Production Speed: 10, 25 and 40 tablets/min (respective linear punch velocity: 38, 96 and 152 mm/s ; respective simulated rotary press speed: 60000, 150000 and 240000 tablets/hour)
- Tooling: Diameter 10 mm R9 concave
- Formula: 98.8% PEARLITOL® 200 GT / 1.2% magnesium stearate
Storage & Handling
- Shelf Life
- 5 years
- Storage Information
- Storage conditions - The product durability may vary according to packaging type and manufacturing plant. Proper information is shown on labeling and CoA.
- Storage conditions for PACK material - We recommend to preserve the product in its unopened original packaging, preferably protected from wide variations in temperature and humidity. Upon opening, use the product as quickly as possible to prevent moisture regain.
- Expiry date of the packed product - Manufacturing date + 5 years, in its unopened packaging.

