Knowde Enhanced TDS
Identification & Functionality
- Country of Origin
- Ingredient Name
- Food Ingredients Functions
- Ingredients
- Modified Maize Starch
- Technologies
- Product Families
- Product Label
Package labeling Cold swelling hydroxypropyl distarch phosphatePackage labeling US Food starch modifiedIngredients [Listed in Descending Order] Modified starch Ingredients US [Listed in Descending Order] 100% (ds) Modified food starch
Features & Benefits
- Labeling Claims
- Food Ingredients Features
- Functionality
- Granular cold water swelling starch with good dispersion properties
- instant viscosity without cooking (fast hydration)
- short, smooth, creamy texture comparable to cook-up starches
- Very good resistance to acid, heat and shear
- Very good freeze-thaw stability
- Very high paste clarity
- Bake stable
- Excellent shelf life
Applications & Uses
- Application
This product is a cold-water swelling starch that possesses many of the desired characteristics of premium cook-up starches without heating. CWS starches are produced with a unique spray-cooking technology that produces starch with intact granules, resulting in performance comparable to cook-up counterparts. Starches produced with this process are uniformly cooked with a minimum amount of shear and heat damage. This extremely versatile starch is recommended for medium to severe cold manufacturing processes (such as salad dressings, fruit fillings and toppings) where rapid hydration, high process tolerance, excellent texture, and long shelf life are required.
Properties
- Nutritional Information (per 100 gm)
- Note for Nutritional Data
- The list comprises relevant nutritional components only.
- Values are calculated based on the average of product specifications. In those cases where only a minimum or a maximum value is specified, these values were taken respectively.
- The EU column lists the nutritional values in accordance with Regulation (EU) n° 1169/2011 on food information to consumers.
- The US column lists the nutritional values in accordance with Code of Federal Regulations (CFR 21).
- The Japan column lists the nutritional values in accordance with the Japanese Legislation.
- The energy values may differ per region because of different calculations.
| Value | Units | Test Method / Conditions | |
| Energy | 373.1 | kcal/100g | — |
| Energy | 1585 | kJ/100g | — |
| Protein Content | 0.3 | g/100g | — |
| Carbohydrates | 93 | g/100g | — |
| Sugar Content | 0 | g/100g | — |
| Added Sugars | 0 | g/100g | — |
| Starch | 93 | g/100g | — |
| Dietary Fiber | 0 | g/100g | — |
| Fat Content | 0.1 | g/100g | — |
| Saturated Fat | 0 | g/100g | — |
| Trans Fat | 0 | g/100g | — |
| Cholesterol | 0 | mg/100g | — |
| Sodium Content | 10 | mg/100g | — |
| Salt | 25 | mg/100g | — |
| Calcium Content | 7 | mg/100g | — |
| Potassium Content | 5 | mg/100g | — |
| Iron Content | 0.2 | mg/100g | — |
| Vitamin C | 0 | mg/100g | — |
| Vitamin D | 0 | mcg/100g | — |
| Water Content | 7 | g/100g | — |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Dietary Information
Suitable for Certified Halal Yes Yes Kosher Yes Yes Lacto-vegetarian Yes No Ovo-vegan Yes No Vegan Yes No Vegetarian Yes No - GMO Statement
For its operations in Europe, Cargill complies with the EU GMO requirements as principally laid down under EC Regulation No 1829/2003 on 'genetically modified food and feed' and EC Regulation No 1830/2003 on 'the traceability and labeling of food and feed products produced from GMO's'. By ensuring the supply of conventional ingredients in the EU, Cargill thus ensures that there is no need to label its products under either 1829/2003 or 1830/2003.
- Legal Requirements
- REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 October 2004 on materials and articles intended to come into contact with food (as amended)
- REGULATION (EC) No 852/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 April 2004 on the hygiene of foodstuffs (as amended)
- REGULATION (EC) NO 396/2005 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin (as amended)
- COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (as amended)
- Where manufactured in the EU this product is in compliance with Regulation (EC) 1333/2008 and subsequent amendments - on food additives.
- Regulation 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No. 1333/2008.
- Current Food Chemical Codex
- Current JECFA Monograph
- This product is in compliance with : FDA 21 CFR § 172.892, Food starch-modified
- REGULATION (EC) No 178/2002 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (as amended)
Technical Details & Test Data
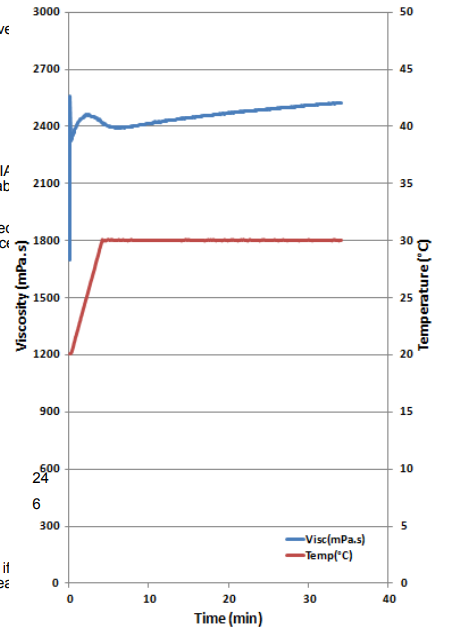
- Test Data
Packaging & Availability
- Packaging Type
- Standard Packaging
Paper Bags
Storage & Handling
- Recommended Storage Conditions
- Store inside, under dry conditions
- Minimum remaining shelf life after delivery (months) : 6
- The specified shelf life can only be guaranteed for this product if the above mentioned recommended storage conditions are respected. For products delivered in bulk, there should be a clean storage (and circulation) system, protected from any potential contamination.

