Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- INCI Name
- Agrochemical Functions
- CASE Ingredients Functions
- Cosmetic Ingredients Functions
- Plastics & Elastomers Functions
- CAS No.
- 112926-00-8
- EC No.
- 601-214-2
- Technologies
- Product Families
Features & Benefits
- Benefit Claims
- Agrochemicals Features
- CASE Ingredients Features
- Materials Features
- Product Features
- Hydrophobic component for thickening and reinforcement of RTV-1 K silicone sealants
- Improves shelf-life of silicone sealants
- Water resistant, hydrophobising of liquid systems
- Rheology control of (complex) liquid systems
- Improvement and maintenance of free flow and anticaking characteristics of powders
- Product Features
AEROSIL® fumed silica such as hydrophilic AEROSIL® 150 is used as a thickening and reinforcing thixotropic filler in silicone sealants. These hydrophilic fumed silica products have freely accessible silanol groups (Si-OH) on their surface causing moisture absorption from environment. However, moisture absorption reduces the shelf-life of the silicone sealants, especially of moisture sensitive neutral alkoxy formulations. Hydrophobization with DDS eliminates the silanol groups and turns the hydrophilic surface of the fumed silica to hydrophobic, for example in AEROSIL® R 972.
Since the moisture adsorption is significantly reduced, AEROSIL® R 972 is the optimum silica product for long shelf-life of moisture sensitive silicone sealant formulations (see Figure 1).
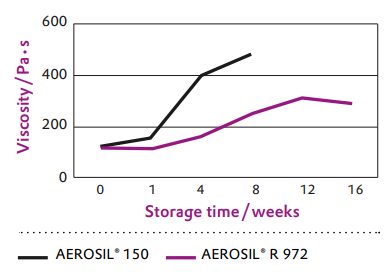
Figure 1 Shelf-life in alcoxy silicone sealants
At first glance, hydrophilic and hydrophobic fumed silica are identical - both are fine white powders. The fundamental difference becomes clear, when they are dispersed in water: while hydrophilic products are completely wetted by water, hydrophobic products do not mix with water at all and remain floating on its surface. This water-repellent behavior is caused by the organic groups anchored at the surface of the fumed silica.
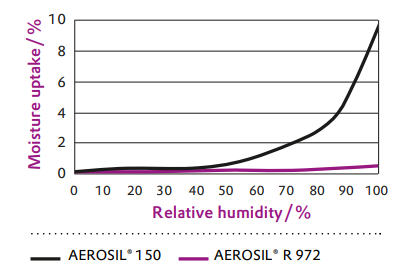
Figure 2 Moisture uptake at room temperature
AEROSIL® R 972 is a fumed silica aftertreated with DDS (dimethyldichlorosilane). An impressive example of the altered surface properties is the absorption of moisture (see Figure 2).Thanks to the nonpolar surface of AEROSIL® R 972 compared with hydrophilic AEROSIL® 150 (a product with no surface treatment), the moisture absorption is significantly reduced.
Applications & Uses
- Markets
- Applications
- Compatible Polymers & Resins
- Plastics & Elastomers End Uses
- Plastics & Elastomers Processing Methods
Properties
- Appearance
- Fluffy white powder
- Physico-Chemical Properties
- Note
1 - According to DIN 9277
² - According to DIN EN ISO 787/11, JIS K 5101/20 (not sieved)
3 - According to DIN EN ISO 787/2, ASTM D 280, JIS K 5101/23
4 - According to DIN EN ISO 3262-20, ASTM D 1208, JIS K 5101/24
5 - According to DIN EN ISO 787/9, ASTM D 1208, JIS K 5101/26
6 - According to DIN EN ISO 787/18, JIS K 5101/22
7 - Based on dried substance (2 hours at 105 °C)
8 - Based on ignited substance (2 hours at 1000 °C)
9 - Special moisture-protective packaging
10 - in water: methanol = 1:1
11 - HCI-content is a part of ignition loss
12 - Packaging of densed material: 20 kg- Note
* ex plant
** Narrower pH possible, depending on region.
| Value | Units | Test Method / Conditions | |
| Unit Weight (Netto) | 10 | kg | — |
| HCI Content⁸,¹¹ | max. 0.050 | wt % | — |
| TiO₂ Content⁸ | max. 0.030 | wt % | — |
| Fe₂O₃ Content⁸ | max. 0.010 | wt % | — |
| Al₂O₃ Content⁸ | max. 0.050 | wt % | — |
| SiO₂ Content⁸ | min. 99.8 | wt % | — |
| Carbon Content | 0.6 - 1.2 | wt % | — |
| pH Value⁵ (4%, Aqueous dispersion) | 3.6 - 5.5 10 | wt % | — |
| Loss on Ignition⁴,⁷ (2h at 1000°C) | max. 2.0 | wt % | — |
| Loss on Drying³ (2h at 105°C, when leaving the plant) | max. 0.5 | wt % | — |
| Tamped Density² | 50 | g/l | — |
| BET Surface Area (BET¹) | 90 - 130 | m²/g | — |
| Behavior Towards Water | Hydrophilic | — | — |
Regulatory & Compliance
- Certifications & Compliance
Technical Details & Test Data
- AEROSIL® Fumed Silica
Four different types of oils used in cable gels were selected for the tests:
- Mineral oil Drakeol® 35 (Penreco, USA)
- Polybutene Napvis® DE 10 (H&R, UK)
- Silicone oil Baysilon® M 1000 (Bayer, Germany)
- Polypropylene glycol (molar mass ~700 g/mol), (Aldrich, USA)
Four of the five AEROSIL® grades tested were hydrophobic because prevention of water penetration to the optical fiber is important. Using a laboratory disperser (Cowles disk, d = 5 cm), the silicas were first thoroughly wetted into the various oils at 6% and 12% at 1000 rpm and then dispersed for 5 minutes at 3000 rpm. The air bubbles introduced into the system by the dispersion process were removed by vacuum.
Rheological measurements
- The Haake RV 20 tester using the CV 20 N measuring system was utilized for the rheological measurements. Rotational measurements were obtained using PK 20.4° and PK 30.4° cones and oscillation measurements were obtained using the Q 30 plate (clearance 1 mm) with a deformation of 5 % at a constant temperature of 25°C. The following ramp function in the rotational measurements was determined after initial shearing (18 sec. at 1 s-1 and 18 sec. at rest).
- In the first test, the shear rate was increased linearly within 2 minutes from approx. 0.3 s-1 to 10 s-1, held constant for 1 minute at 10 s-1, and decreased linearly within 2 minutes from 10 s-1 to approx. 0.3 s-1.
- The flow curve (down) was used to calculate each yield point in accordance with the Casson regression model; the viscosi- ties were also calculated at 2.5 s-1 using the flow curve down).
- In a second test sequence (to determine the recovery of viscosity) the substances were first subjected to shearing at 1 s-1 for 0.5 min., then at 100 s-1 for 0.5 min. and again at 1 s-1 for 0.5 min. The oscillation measurements were carried out as a function of frequency. To do this, the frequency was increased from 0.1 Hz to 9.8 Hz at a constant deformation of 5%. These measurements were used to calculate the loss and storage module.
Rotational and oscillatory measurements
- The measured yield points and viscosities of the various samples are shown in Tables 1 and 2.
- In the case of AEROSIL® 200, despite a 12% concentration, hardly any thickening and thixotropic effect was noticeable in the polar polypropylene glycol. The highest yield points and viscosities were observed when the hydrophobic grade AEROSIL® R 202 was used.
Table 1 - Yield points and viscosities of Drakeol 35 (mineral oil) and Baysilon M 1000 (silicone oil), thickened with 6 % and 12 % different AEROSIL® grades.
Mineral oil Silicone oil Silica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in PasSilica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in Pas6 % AEROSIL® R 972
12 % AEROSIL® R 9724.0
93.04.0
66.06 % AEROSIL® R 972
12 % AEROSIL® R 9721.0
1.04.0
17.0Mineral oil 0.0 0.135 Silicone oil 0.0 0.960 Table 2 - Yield points and viscosities of Napvis DE 10 (polybutene) and polypropylene glycol, thickened with 6 % and 12 % different AEROSIL® grades
Polybutene oil Polypropylene glycol
Silica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in PasSilica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in Pas6 % AEROSIL® R 972 4.0 66.0 12 % AEROSIL® R 972 0.3 0.7 Polybutene oil 0 25 Polypropylenglycol 0 0.11 The following paragraphs explain the thickening effect of AEROSIL®, which depends on the polarity of the oil, as well as the mechanisms of thickening and thixotropy of the different silicas in liquids. The specific test results are also diskussed.
- The thickening and thixotropic effect result from the forma- tion of a threedimensional network of AEROSIL® particles in the system. With the addition of shearing forces (shak- ing or stirring), the network is broken down, depending on the intensity and duration of the stress, and the viscosity is reduced accordingly. At rest, the network reforms and the system recovers to its original viscosity.
- The interactions between the silanol groups of different AEROSIL® particles are responsible for the formation and stability of the network. The hydrogen bonds between the AEROSIL® particles have their full effect in nonpolar liquids such as hydrocarbons or polydimethyl siloxanes. As soon as the liquid molecules exhibit a greater or lesser affinity to the silanol groups as determined by their structure, solvation of the AEROSIL® particles occurs and with it destabilization of the spatial network. For this reason the thickening of polar liquids such as ethanol or water, or in this case polypropyl- ene glycol, is only possible with relatively large quantities of hydrophilic AEROSIL® grades.
- If we now compare the thickening effect of the hydrophobic AEROSIL® grades in the three non-polar oils, the highest yield points and viscosities are achieved in all three oils (mineral, silicone and polybutene oil) when AEROSIL® R 805 is used, while AEROSIL® R 972 exhibits only a minimal thickening effect.
- It is only with a concentration of 12% that AEROSIL® R 972 comes close to a yield point of 93 Pa in mineral oil, which is comparable to the yield point of 92 Pa at a 6% concentra- tion of AEROSIL® R 805. In comparison to AEROSIL® R 972, AEROSIL® R 974 is more effective in terms of thickening in all three non-polar oils. This is due to its greater surface area of 170 m²/g as compared to 110 m2/g for AEROSIL® R 972 with the same functional surface groups. The physical mixing of AEROSIL® 200 and AEROSIL® R 202 in a ratio of 1:1 also pro- duces very high yield points and viscosities in mineral, silicone and polybutene oils.
The following mechanism can be used to explain the rheo- logical effect of hydrophobic AEROSIL® grades.
- The functional groups on the surfaces of AEROSIL® R 805, AEROSIL® R 202, AEROSIL® R 972 and AEROSIL® R 974 as shown in Figure 3 interact both with each other and with the binding agent, creating especially Van-der-Waals-linkages among the silica particles. As before, a three-dimensional network structure results which increases the viscosity and imparts thixotropic characteristics to the system. The remain- ing silanol groups support this mechanism in non-polar sys- tems by forming hydrogen bonds, whereas in polar systems they are shielded by the functional groups, particularly those of AEROSIL® R 805 and AEROSIL® R 202.
- This model explains the superior thickening effect of AEROSIL® R 202 and AEROSIL® R 805 as compared with AEROSIL® 200, AEROSIL® R 972 and AEROSIL® R 974 in the polar polypropylene glycol and other polar systems such as epoxy resins and vinyl ester resins (3, 4). The methyl groups on the surface of AEROSIL® R 972 and AEROSIL® R 974 are probably too short to achieve the same effect as those of the larger functional groups of AEROSIL® R 202 and AEROSIL® R 805.
- The current results show that the measured viscosities and yield points of the four different oils vary significantly when different AEROSIL® grades are added. For example, it is necessary to have 12% AEROSIL® R 972 and only 6 % AEROSIL® R 805 to achieve the same yield point in mineral oil. Oscillation measurements were used to determine if there are additional explanations for these differences other than the rheology models already diskussed. These measurements make it possible to demonstrate that, only by the addition of AEROSIL® fumed silica to the various oils can visco-elastic properties result.
- Table below shows the measured loss and storage modulus for the mineral and silicone oil and the loss factor tan d calculated at an angle velocity of 10 s-1. Loss modulus G" is a measure of the viscous component, whereas storage modulus G' is a measure of the elastic component in the system. For the sake of clarity, the two moduli have been calculated at a constant angle velocity of 10 s-1 and not shown as a function of the angle velocity. Loss factor tan d is the ratio G" / G'; if it is greater than 1, the viscous components in the system are greater, and if it is smaller than 1, the elastic components are greater.
- The test results in Table show, that when the AEROSIL® grades are used, different viscoelastic properties can be obtained in the thickened oils. Figure shows that there is a good correlation between the elastic properties and the yield points of the thickened min- eral and silicone oils. In these oils, as the storage modulus G' increases, so does the yield point.
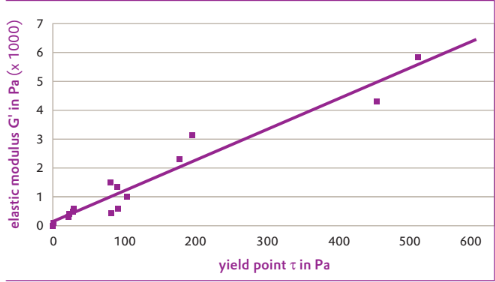
Correlation of elastic modulus and yield point of oils thickened with various AEROSIL® grades.
- Beyond that, the loss factors tan d are less than 1 for all sam- ples with high yield points and greater than 1 for all samples with low yield points. Based on these measurements the following additional mechanism is being suggested for rheological effect. When the thickened oil is suddenly subjected to shearing forces, the three-dimensional network of silica particles reacts because of its elastic properties and absorbs the deforming energy.
- Initially, there is resistance against being deformed; the system indicates solid-like behavior. For elastic like behav- iour to occur, we must assume that the hydrophilic AEROSIL® particles exhibit hydrogen bonding while the hydrophobic AEROSIL® particles react due to Van-der-Waals attractions. Furthermore, we must consider also steric circumstances.
Table - Elastic modulus G', loss modulus G" and tan d of Drakeol 35 (mineral oil) and Baysilon M 1000 (silicone oil) at 10 s-1, thickened with 6% and 12% different AEROSIL® grades.
Mineral oil Silicone oil Silica grade and
concentrationElastic modulus
G' in Pa lossModulus
G'' in Patan δ
(calculated)Silica grade and
concentrationElastic modulus
G' in Pa lossModulus
G'' in Patan δ
(calculated)6 % AEROSIL® R 972
12 % AEROSIL® R 972110.0
590.0100.0
610.00.91
1.036 % AEROSIL® R 972
12 % AEROSIL® R 9725.0
50.040.0
105.08.00
2.10At rates of deformation higher than the yield point, the silica particles move in the shear field and disarrange each other, assuming a configuration that is adverse to a normal thermo- dynamic conformation. Under these higher shear rates, the thixotropic oil indicates predominantly viscous behavior and begins to flow. After removing the shearing deformation, the elastic properties of the visco-elastic system dominate. The energy, which is absorbed by the elastic deformation, is released and the three-dimensional network of silica particles forms again. The yield point recovers almost to its original value.
The mixture with 12 % AEROSIL® R 972 and 6 % AEROSIL® R 805 in the mineral oil are compared below in greater detail. The yield points are almost identical at 93 Pa and 92 Pa, which means that AEROSIL® R 805 is about twice as effective as AEROSIL® R 972 in terms of yield point. This is because the mineral oil thickened with 6% AEROSIL® R 805 has a higher storage modulus G' of 1350 Pa compared with 590 Pa for 12% AEROSIL® R 972. At the same time, loss factor tan d is 0.45 for the sample with AEROSIL® R 805 and 1.03 for AEROSIL® R 972.
Obviously, the higher elasticity of the mixture thickened with AEROSIL® R 805 is caused by the octyl groups on the surface. As diskussed earlier, these groups react due to Van-der-Waals forces. The methyl groups on the surface of AEROSIL® R 972 are obviously too short to achieve the same large particle- particle interactions. With this model, we can explain why AEROSIL® R 805 is about twice as effective as AEROSIL® R 972 in terms of yield point.
Low and high shear tests carried out by means of rotational measurements as a function of time correlate with these results. As shown in Figure below, the recovery in the mineral oil thickened with 6% AEROSIL® R 805 was almost 100% due to its high level of elastic components and "only" about 90% in the mineral oil thickened with 12% AEROSIL® R 972. This example also shows that recovery takes place within a few tenths of a second in both substances.
Problem-free extrusion of the cable gels into the cable jacket requires both complete and rapid recovery of the original viscosity after the cable has been subjected to shearing forces. Both properties, which in effect characterize the thixotropic properties of cable gels, can be obtained using AEROSIL® fumed silica.
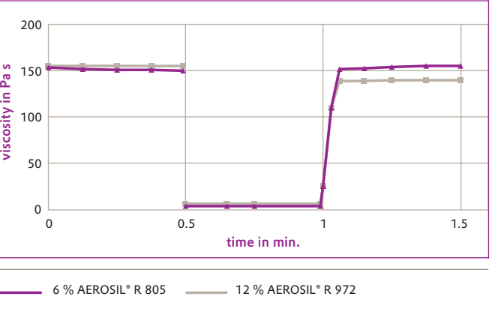
Viscosity rebounding after shearing deformation of mineral oils, thickened with 6% AEROSIL® R 805 and 12% AEROSIL® R 972
Electrical measurements
The methods used to measure the dielectric properties are described in (8). The dielectric constant, as measured at 1 MHz and 25°C, was 2.17 for mineral oil, 2.19 for polybutene oil and 2.67 for silicone oil. When the AEROSIL® grades tested here are used in these oils the dielectric constant remains practically unchanged. The dielectric loss factor tan d was less than 0.001 in all the samples. Good dielectric properties are particularly important for filler compounds used in copper cable (8).
- Test Methods
Four different types of oils used in cable gels were selected for the tests:
- Mineral oil Drakeol® 35 (Penreco, USA)
- Polybutene Napvis® DE 10 (H&R, UK)
- Silicone oil Baysilon® M 1000 (Bayer, Germany)
- Polypropylene glycol (molar mass ~700 g/mol), (Aldrich, USA)
Four of the five AEROSIL® grades tested were hydrophobic because prevention of water penetration to the optical fibre is important. Using a laboratory disperser (Cowles disc, d = 5 cm), the silicas were first thoroughly wetted into the various oils at 6% and 12% at 1000 rpm and then dispersed for 5 minutes at 3000 rpm. The air bubbles introduced into the system by the dispersion process were removed by vacuum.
Rheological measurements
- The Haake RV 20 tester using the CV 20 N measuring system was utilized for the rheological measurements. Rotational measurements were obtained using PK 20.4° and PK 30.4° cones and oscillation measurements were obtained using the Q 30 plate (clearance 1 mm) with a deformation of 5 % at a constant temperature of 25°C. The following ramp function in the rotational measurements was determined after initial shearing (18 sec. at 1 s-1 and 18 sec. at rest).
- In the first test, the shear rate was increased linearly within 2 minutes from approx. 0.3 s-1 to 10 s-1, held constant for 1 minute at 10 s-1, and decreased linearly within 2 minutes from 10 s-1 to approx. 0.3 s-1.
- The flow curve (down) was used to calculate each yield point in accordance with the Casson regression model; the viscosi- ties were also calculated at 2.5 s-1 using the flow curve down).
- In a second test sequence (to determine the recovery of viscosity) the substances were first subjected to shearing at 1 s-1 for 0.5 min., then at 100 s-1 for 0.5 min. and again at 1 s-1 for 0.5 min. The oscillation measurements were carried out as a function of frequency. To do this, the frequency was increased from 0.1 Hz to 9.8 Hz at a constant deformation of 5%. These measurements were used to calculate the loss and storage module.
Rotational and oscillatory measurements
- The measured yield points and viscosities of the various samples are shown in Tables 1 and 2.
- In the case of AEROSIL® 200, despite a 12% concentration, hardly any thickening and thixotropic effect was noticeable in the polar polypropylene glycol. The highest yield points and viscosities were observed when the hydrophobic grade AEROSIL® R 202 was used.
Table 1 - Yield points and viscosities of Drakeol 35 (mineral oil) and Baysilon M 1000 (silicone oil), thickened with 6 % and 12 % different AEROSIL® grades.
Mineral oil Silicone oil Silica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in PasSilica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in Pas6 % AEROSIL® R 972
12 % AEROSIL® R 9724.0
93.04.0
66.06 % AEROSIL® R 972
12 % AEROSIL® R 9721.0
1.04.0
17.0Mineral oil 0.0 0.135 Silicone oil 0.0 0.960 Table 2 - Yield points and viscosities of Napvis DE 10 (polybutene) and polypropylene glycol, thickened with 6 % and 12 % different AEROSIL® grades
Polybutene oil Polypropylene glycol
Silica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in PasSilica grade and
concentrationYield point
in PaViscosity at
2.5 s-1 in Pas6 % AEROSIL® R 972 4.0 66.0 12 % AEROSIL® R 972 0.3 0.7 Polybutene oil 0 25 Polypropylenglycol 0 0.11 The following paragraphs explain the thickening effect of AEROSIL®, which depends on the polarity of the oil, as well as the mechanisms of thickening and thixotropy of the different silicas in liquids. The specific test results are also discussed.
- The thickening and thixotropic effect result from the forma- tion of a threedimensional network of AEROSIL® particles in the system. With the addition of shearing forces (shak- ing or stirring), the network is broken down, depending on the intensity and duration of the stress, and the viscosity is reduced accordingly. At rest, the network reforms and the system recovers to its original viscosity.
- The interactions between the silanol groups of different AEROSIL® particles are responsible for the formation and stability of the network. The hydrogen bonds between the AEROSIL® particles have their full effect in nonpolar liquids such as hydrocarbons or polydimethyl siloxanes. As soon as the liquid molecules exhibit a greater or lesser affinity to the silanol groups as determined by their structure, solvation of the AEROSIL® particles occurs and with it destabilisation of the spatial network. For this reason the thickening of polar liquids such as ethanol or water, or in this case polypropyl- ene glycol, is only possible with relatively large quantities of hydrophilic AEROSIL® grades.
- If we now compare the thickening effect of the hydrophobic AEROSIL® grades in the three non-polar oils, the highest yield points and viscosities are achieved in all three oils (mineral, silicone and polybutene oil) when AEROSIL® R 805 is used, while AEROSIL® R 972 exhibits only a minimal thickening effect.
- It is only with a concentration of 12% that AEROSIL® R 972 comes close to a yield point of 93 Pa in mineral oil, which is comparable to the yield point of 92 Pa at a 6% concentra- tion of AEROSIL® R 805. In comparison to AEROSIL® R 972, AEROSIL® R 974 is more effective in terms of thickening in all three non-polar oils. This is due to its greater surface area of 170 m²/g as compared to 110 m2/g for AEROSIL® R 972 with the same functional surface groups. The physical mixing of AEROSIL® 200 and AEROSIL® R 202 in a ratio of 1:1 also pro- duces very high yield points and viscosities in mineral, silicone and polybutene oils.
The following mechanism can be used to explain the rheo- logical effect of hydrophobic AEROSIL® grades.
- The functional groups on the surfaces of AEROSIL® R 805, AEROSIL® R 202, AEROSIL® R 972 and AEROSIL® R 974 as shown in Figure 3 interact both with each other and with the binding agent, creating especially Van-der-Waals-linkages among the silica particles. As before, a three-dimensional network structure results which increases the viscosity and imparts thixotropic characteristics to the system. The remain- ing silanol groups support this mechanism in non-polar sys- tems by forming hydrogen bonds, whereas in polar systems they are shielded by the functional groups, particularly those of AEROSIL® R 805 and AEROSIL® R 202.
- This model explains the superior thickening effect of AEROSIL® R 202 and AEROSIL® R 805 as compared with AEROSIL® 200, AEROSIL® R 972 and AEROSIL® R 974 in the polar polypropylene glycol and other polar systems such as epoxy resins and vinyl ester resins (3, 4). The methyl groups on the surface of AEROSIL® R 972 and AEROSIL® R 974 are probably too short to achieve the same effect as those of the larger functional groups of AEROSIL® R 202 and AEROSIL® R 805.
- The current results show that the measured viscosities and yield points of the four different oils vary significantly when different AEROSIL® grades are added. For example, it is necessary to have 12% AEROSIL® R 972 and only 6 % AEROSIL® R 805 to achieve the same yield point in mineral oil. Oscillation measurements were used to determine if there are additional explanations for these differences other than the rheology models already discussed. These measurements make it possible to demonstrate that, only by the addition of AEROSIL® fumed silica to the various oils can visco-elastic properties result.
- Table below shows the measured loss and storage modulus for the mineral and silicone oil and the loss factor tan d calculated at an angle velocity of 10 s-1. Loss modulus G" is a measure of the viscous component, whereas storage modulus G' is a measure of the elastic component in the system. For the sake of clarity, the two moduli have been calculated at a constant angle velocity of 10 s-1 and not shown as a function of the angle velocity. Loss factor tan d is the ratio G" / G'; if it is greater than 1, the viscous components in the system are greater, and if it is smaller than 1, the elastic components are greater.
- The test results in Table show, that when the AEROSIL® grades are used, different viscoelastic properties can be obtained in the thickened oils. Figure shows that there is a good correlation between the elastic properties and the yield points of the thickened min- eral and silicone oils. In these oils, as the storage modulus G' increases, so does the yield point.

Correlation of elastic modulus and yield point of oils thickened with various AEROSIL® grades.
- Beyond that, the loss factors tan d are less than 1 for all sam- ples with high yield points and greater than 1 for all samples with low yield points. Based on these measurements the following additional mechanism is being suggested for rheological effect. When the thickened oil is suddenly subjected to shearing forces, the three-dimensional network of silica particles reacts because of its elastic properties and absorbs the deforming energy.
- Initially, there is resistance against being deformed; the system indicates solid-like behaviour. For elastic like behav- iour to occur, we must assume that the hydrophilic AEROSIL® particles exhibit hydrogen bonding while the hydrophobic AEROSIL® particles react due to Van-der-Waals attractions. Furthermore, we must consider also steric circumstances.
Table - Elastic modulus G', loss modulus G" and tan d of Drakeol 35 (mineral oil) and Baysilon M 1000 (silicone oil) at 10 s-1, thickened with 6% and 12% different AEROSIL® grades.
Mineral oil Silicone oil Silica grade and
concentrationElastic modulus
G' in Pa lossModulus
G'' in Patan δ
(calculated)Silica grade and
concentrationElastic modulus
G' in Pa lossModulus
G'' in Patan δ
(calculated)6 % AEROSIL® R 972
12 % AEROSIL® R 972110.0
590.0100.0
610.00.91
1.036 % AEROSIL® R 972
12 % AEROSIL® R 9725.0
50.040.0
105.08.00
2.10At rates of deformation higher than the yield point, the silica particles move in the shear field and disarrange each other, assuming a configuration that is adverse to a normal thermo- dynamic conformation. Under these higher shear rates, the thixotropic oil indicates predominantly viscous behaviour and begins to flow. After removing the shearing deformation, the elastic properties of the visco-elastic system dominate. The energy, which is absorbed by the elastic deformation, is released and the three-dimensional network of silica particles forms again. The yield point recovers almost to its original value.
The mixture with 12 % AEROSIL® R 972 and 6 % AEROSIL® R 805 in the mineral oil are compared below in greater detail. The yield points are almost identical at 93 Pa and 92 Pa, which means that AEROSIL® R 805 is about twice as effective as AEROSIL® R 972 in terms of yield point. This is because the mineral oil thickened with 6% AEROSIL® R 805 has a higher storage modulus G' of 1350 Pa compared with 590 Pa for 12% AEROSIL® R 972. At the same time, loss factor tan d is 0.45 for the sample with AEROSIL® R 805 and 1.03 for AEROSIL® R 972.
Obviously, the higher elasticity of the mixture thickened with AEROSIL® R 805 is caused by the octyl groups on the surface. As discussed earlier, these groups react due to Van-der-Waals forces. The methyl groups on the surface of AEROSIL® R 972 are obviously too short to achieve the same large particle- particle interactions. With this model, we can explain why AEROSIL® R 805 is about twice as effective as AEROSIL® R 972 in terms of yield point.
Low and high shear tests carried out by means of rotational measurements as a function of time correlate with these results. As shown in Figure below, the recovery in the mineral oil thickened with 6% AEROSIL® R 805 was almost 100% due to its high level of elastic components and "only" about 90% in the mineral oil thickened with 12% AEROSIL® R 972. This example also shows that recovery takes place within a few tenths of a second in both substances.
Problem-free extrusion of the cable gels into the cable jacket requires both complete and rapid recovery of the original viscosity after the cable has been subjected to shearing forces. Both properties, which in effect characterise the thixotropic properties of cable gels, can be obtained using AEROSIL® fumed silica.

Viscosity rebounding after shearing deformation of mineral oils, thickened with 6% AEROSIL® R 805 and 12% AEROSIL® R 972
Electrical measurements
The methods used to measure the dielectric properties are described in (8). The dielectric constant, as measured at 1 MHz and 25°C, was 2.17 for mineral oil, 2.19 for polybutene oil and 2.67 for silicone oil. When the AEROSIL® grades tested here are used in these oils the dielectric constant remains practically unchanged. The dielectric loss factor tan d was less than 0.001 in all the samples. Good dielectric properties are particularly important for filler compounds used in copper cable (8).
Packaging & Availability
- Packaging Type
- Regional Availability
- Packaging Information
AEROSIL® R 972 is supplied in multiple layer 10 kg bags.
Storage & Handling
- Shelf Life
- 2 years
- Storage Conditions
Recommend to store the product in closed containers under dry conditions and to protect the material from volatile substances. AEROSIL® R 972 should be used within 2 years after
production.

