Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- INCI Name
- Cosmetic Ingredients Functions
- Pharma & Nutraceuticals Functions
- CAS No.
- 17465-86-0
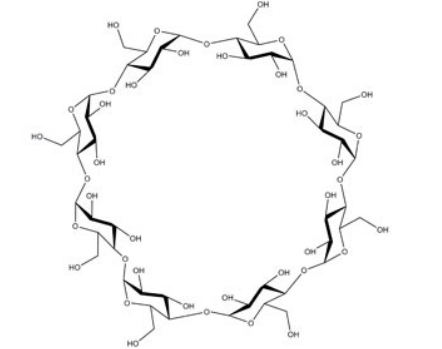
- Molecular formula
- C₄₈H₈₀O₄₀
- EC No.
- 241-482-4
- Product Families
- Chemical Structure
Features & Benefits
- Benefit Claims
- Labeling Claims
Applications & Uses
- Markets
- Application Details
Solubilization, stabilization and delivery of large active ingredients, e.g. steroids, lipids
Enhancement of both oral bioavailability and taste masking of pharmaceutical active ingredients; enabling of delayed release.- Applications
- CAVAMAX® Cyclodextrins & CAVASOL® Cyclodextrin Derivatives
- Personal Care
- Pharma
Properties
- Typical Properties
- Microbiological Values
- Specifications
- Properties
- DMF Type IV, No 11375
- White powder
- Pharmaceutical grade: Content min. 98.0%, complies with current USP/NF monographs specifications.
| Value | Units | Test Method / Conditions | |
| Molecular Weight | 1297 | g/mol | — |
| Bulk Density | 400 - 700 | kg/m³ | — |
| Solubility in Water (at 20°C) | 180.1 | g/l | OECD 105 |
| Value | Units | Test Method / Conditions | |
| Escherichia coli | 0 | per 10g | Microbiological photometric test |
| Microorganisms | max. 1000 | per gram | Microbiological photometric test |
| Molds and Yeasts | max. 100 | per gram | Microbiological photometric test |
| Salmonella | 0 | per 10g | Microbiological photometric test |
| Value | Units | Test Method / Conditions | |
| Absorbance (10% solution, at 420nm) | max. 0.2 | — | USP/NF |
| Cyclodextrin Content | 98.0 - 102.0 | % | HPLC Method |
| Heavy Metals | max. 5 | ppm | USP/NF |
| Loss on Drying | max. 11.0 | % | Halogen dryer |
| Reducing Substances | max. 0.2 | % | PH. EUR. |
| Related Substances | max. 0.5 | % | USP/NF |
| Related Substances (sum) | max. 0.5 | % | USP/NF |
| Residue on Ignition | max. 0.1 | % | USP/NF |
| Specific Rotation ([α]/D20) | 174.0 - 180.0 | Degree | USP/NF |
| Volatile Organic Compounds | max. 50 | ppm | Gas Chromatography |
Regulatory & Compliance
- Certifications & Compliance
- Chemical Inventories
- Grade
Packaging & Availability
- Country Availability
- Regional Availability
- Packaging Information
Units of 20 kg
Storage & Handling
- Storage Information
Storage at room temperature in sealed containers under dry conditions is recommended. CAVAMAX® W8 PHARMA has a shelf life of at least 36 months when stored inunbroken original packaging in dry storage areas.

