Knowde Enhanced TDS
Identification & Functionality
- Chemical Name
- Pharma & Nutraceuticals Functions
- CAS No.
- 9006-65-9
- EC No.
- 618-433-4
- Technologies
- Chemical Structure
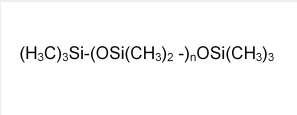
Features & Benefits
- Labeling Claims
Applications & Uses
- Applications
Pharma
- Application Details
SILFAR® 100 is used predominantly in the pharmaceutical sector e.g. as an basic raw material in phamaceutical formulations.
Properties
- Odor
- Odorless
- Typical Properties
- Properties
SILFAR® 100 complies with the requirements set down in the monographs Dimeticone (EP) and "Dimethicone" (USP / NF). Wacker explicitly rules out the use of SILFAR® 100 for parenteral applications.
| Value | Units | Test Method / Conditions | |
| Acidity | max. 5 | mg/kg | Direct Titration Method |
| Density (at 25°C) | 0.962 - 0.970 | g/cm³ | DIN 51757 |
| Kinematic Viscosity (at 25°C) | 95 - 105 | mm²/s | DIN 53018 |
| Refractive Index (at 25°C) | 1.402 - 1.404 | — | — |
| Solidification Point | approx. -55 | °C | — |
| Volatile Content | max. 0.3 | % | USP |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Quality Standards
Packaging & Availability
- Country Availability
- Regional Availability
Storage & Handling
- Shelf Life
- 720 Days
- Storage Information
Keep container tightly closed. Store container(s) at room temperature in a dry, wellventilated place.

