Knowde Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Ingredient Name
- CASE Ingredients Functions
- Food Ingredients Functions
- Industrial Additives Functions
- Pharma & Nutraceuticals Functions
- Plastics & Elastomers Functions
- CAS No.
- 9006-65-9
- Food Additive Number
- E 900a, INS 900 a
- Ingredients
- Dichlorodimethylsilane, Chlorotrimethylsilane
- EC No.
- 618-433-4
- Technologies
- Product Families
- Chemical Structure
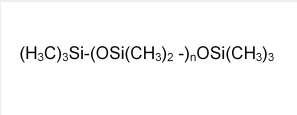
Features & Benefits
- Food Ingredients Features
- Industrial Additives Features
- Materials Features
Applications & Uses
- Applications
- Dosage Form
- Food & Nutrition Applications
- Industrial Additives End Use
- Plastics & Elastomers End Uses
- Application Details
- SILFAR® 350 is used both in the pharmaceutical and food sectors. In the pharmaceutical sector it is used in antiflatulence and antacid preparations. It acts as an antifoam agent to prevent or relieve flatulence in the alimentary canal and forms a protective lining on e.g. the gastric mucous membrane.
- SILFAR® 350 may be used to make tablets and coated tablets. It is often combined with fermentation preparations.
- SILFAR® 350 is also used to control foam in fermentation and downstream processes, such as active-ingredient extraction and solvent recovery, especially when USP/EP compliant products are mandatory.
- In the food sector, SILFAR® 350 is used predominantly to control foam in lipophilic systems (foods rich in oil or fat, e.g. deep-frying oil) or for surface treatment. SILFAR® 350 also serves as the base material in the production of antifoams compliant with the regulations pertaining to food for human consumption.
- Applications
- Food
- Pharma
Properties
- Odor
- Odorless
- Typical Properties
| Value | Units | Test Method / Conditions | |
| Acidity | max. 5 | mg/kg | Direct Titration Method |
| Active Substance | 100 | % | — |
| Density (at 25°C) | 0.965 - 0.973 | g/cm³ | DIN 51757 |
| Flash Point | min. 300 | °C | ISO 2592 |
| Ignition Temperature | 450 | °C | DIN 51794 |
| Kinematic Viscosity (at 25°C) | 333 - 367 | mm²/s | DIN 53019 |
| Refractive Index (at 25°C) | 1.402 - 1.404 | — | — |
| Solidification Point | -50 | °C | — |
| Volatile Content | max. 0.3 | % | USP |
Regulatory & Compliance
- Certifications & Compliance
- Grade
- Quality Standards
- Regulatory status
Use as pharmaceutical excipient:
SILFAR® 350 complies with the requirements set down in the monographs Dimeticone (EP) and "Dimethicone" (USP / NF). Wacker explicitly rules out the use of SILFAR® 350 for parenteral applications.
Direct Food additive:
- SILFAR® 350 is listed in the Regulation (EC) No 1333/2008 Annex II and III as amended by Regulations (EU) Nos 1129/2011 and 1130/2011 as E 900 polydimethylsiloxane. It meets the purity requirements as laid down in Regulation (EU) No. 231/2012 and is therefore approved as direct food additive within the EC under observance of the use restrictions in force for polydimethylsiloxane (E 900).
- SILFAR® 350 is certified according to HACCP principles. Hence, it meets the requirements on food hygiene in accordance with Regulation (EC) No. 852/2004.
Food Contact:
SILFAR® 350 complies with the Recommendation "XV Silicones" of the BfR concerning the manufacture of essential commodities that have contact with food. SILFAR® 350 is listed under PM-Nr. 76721 in Regulation (EU) No. 10/2011 as an additive for plastic materials to come into contact with food and meets the specifications under column (4) and (10). The product is not subject to a specific migration limit (SML). SILFAR® 350 is suitable for use under FDA 21 CFR as follows:
- §175.105 (Adhesives),
- §175.300 (Resinous and polymeric coatings),
- §176.170 (Components of paper and paperboard in contact with aqueous and fatty foods)
- §176.180 (Components of paper and paperboard in contact with dry food).
- §176.200 (Defoaming agents used in coatings)
- §176.210 (Defoaming agents used in the manufacture of paper and paperboard)
- §177.1210 (Closures with sealing gaskets for food containers).
- §177.2800 (Textiles and textile fibers).
- §178.3120 (Animal glue).
- §178.3570 (Lubricants with incidental food contact)
- §178.3910 (Surface lubricants used in the manufacture of metallic articles)
- §181.28 (Release agents)
SILFAR® 350 meets the criteria for an exemption from the requirements of a tolerance when used as an additive in pesticide formulations to be applied to growing crops or raw agricultural commodities after harvest as specified in 40 CR 180.960.
Packaging & Availability
- Country Availability
- Regional Availability
Storage & Handling
- Shelf Life
- 720 Days
- Storage Information
Keep container tightly closed. Store container(s) at room temperature in a dry, wellventilated place.

